A New Vaccine for Dengue and Ongoing Concerns

Pain. Pain so severe that it feels as if your bones have broken. This is how dengue is described and how it earned the common name, “bone break fever”.(1) This week the World Health Organization (WHO) approved Dengvaxia (CYD-TDV) by Sanofi Pasteur, a vaccine for dengue.(2) This new vaccine is potentially a fantastic boon and a terrible mistake.
Dengue “threatens the health of almost 40% of the world’s population making it the most widespread vector borne viral disease.” (3) At least 50 million infections are thought to occur annually, resulting in approximately 500 000 hospitalizations, mainly in children.(18) WHO states that the incidence of dengue has increased 30 fold over the last 50 years. Part of the blame is laid on World War II, where ‘Coincidental transportation’ of the Aedes egypti mosquitos in cargo spread the disease quickly (4)
The disease is spread from person to person through a mosquito vector, (the same mosquito implicated in the spread of Zika). (diagram) The mosquito feeds on an infected human host while the virus is plentiful in the blood, usually 5 to 7 days after the host has contracted the disease. The virus must incubate within the mosquito for about 8 days to grow a strength sufficient to be transmitted. The mosquito then feeds on a second human host who will contract the disease.(5) The mosquito will remain infected for the rest of its life, be up to two weeks. It will feed for every batch of eggs it lays, up to 5 batches of about 70 eggs. (6)
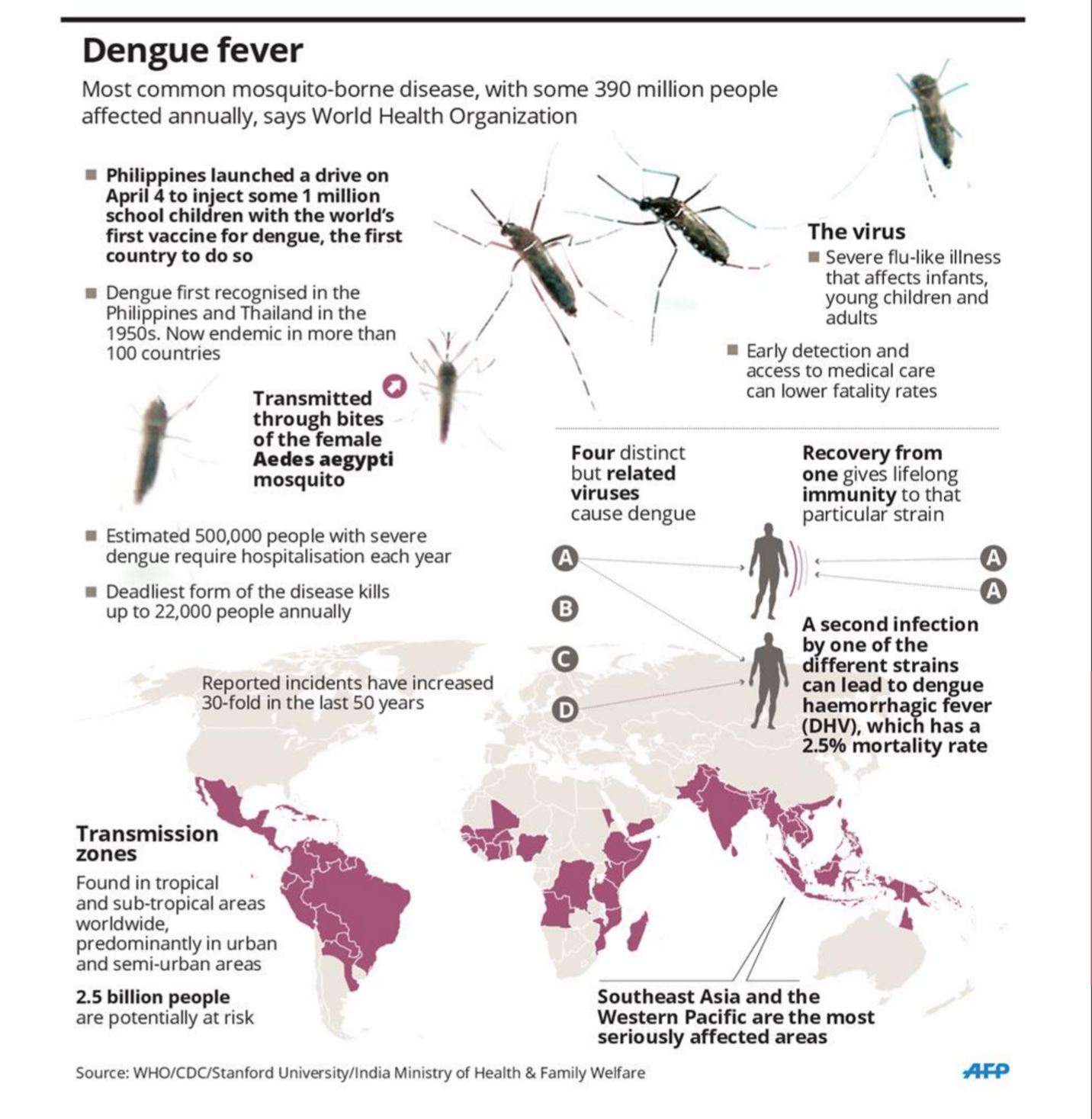
The vaccine was licensed in Mexico (6) in December. It is already being used in the Philippines, and El Salvador. Brazil is also offering the vaccine. (7) Sri Lanka has announced its intention to begin investigations. (8) It is expected, with the World Health Organization’s announcement, that many other countries will also start vaccination programs.
Sanofi Pastuer,(9) a company based in France, claims to have developed a tetravalent (useful against all four serotypes of dengue virus) vaccine for people from the ages of 9-45, though its internal reports suggest problems. (10) It is the first in a group of vaccines that are currently in various stages of testing. Sanofi Pastuer’s vaccine was created from an attenuated virus (live but weakened) that has been genetically modified from a yellow fever virus. (11) It is vitally important to protect against all four strains, as the contraction of a different strain of dengue after the body has built an immunity to one may lead to dengue hemorrhagic fever (DHV) (pictured) which is lethal in 2.5% of cases. (12)

There are still concerns. According to 2012 report on the vaccine funded by Sanofi Pasteur, “efficacy was not shown against DENV2. This lack of efficacy against DENV2, and the fact that DENV2 was the prevalent serotype during the study, diminished the overall vaccine efficacy in this setting.” (20) If the vaccine is not effective against all strains of dengue, then the chance for reinfection and the problems of dengue hemorrhagic fever are exacerbated. This would increase the number of deaths and complications exponentially.
Additionally, about 95% of cases occur in children under 15 years of age and at least 5% of these in infants. The disease appears to attack the very young, and the first peak (in incident rates) occurred from 6 to 9 months of age when levels of maternal antibody had declined, but not disappeared, and the infant was exposed to infection with a serotype different from that which had infected the mother. The second peak occurred in young children who had experienced an earlier, usually mild or subclinical, infection (20)
The second exposure is the potentially harmful one. The child will be immune to the strain with which his mother’s milk has given him antibodies until the age at which he depends less on the milk and more upon outside sources for food. This causes the first peak. If he is then exposed to a strain that he does not have immunity, his immune system may react toward the second pathogen with a hemorrhagic fever. (DHF) which causes internal bleeding and sometimes death. Children who avoid this early disease face the second peak in early childhood. The current vaccine is targeted for older children, starting at the age of nine. Many of the most vulnerable population will be left unguarded.

The development of the vaccine has taken over 20 years. (13) There have been many hurdles to overcome due to “lack of will/interest to invest in platforms that have the potential to become successful vaccines,” (14) The lack of will/interest was because the populations at risk were poor and there was little profit to be made from the creation of the vaccine.
The complexity of the problem of “viral interference and balancing attenuation to produce acceptable tetravalent immunogenicity with minimal reactogenicity (which is) the problem of immunizing against four serotypes”. (15) was also an issue. The problem of multiple strains where exposure to one strain sensitized the body to the other strains made development difficult at best. Stated more simply, “With the second infection, the antibodies sort of recognize the new type of viruses, but not well enough to clear them from the system. Instead of neutralizing the viruses, (the body) actually helps them invade the immune system’s other cells and spread.”(16)
Before vaccine use, dengue was seen as a “potentially fatal viral disease for which treatment is limited to supportive care, and prevention and control are based on mosquito vector control programs.” (17) Mosquito abatement will not be abandoned, as evidenced by this excerpt from Peru Reports.
“Piura has seen over 1,100 confirmed cases of dengue and two deaths in 2015 to date. Peru’s health ministry declared a 90-day state of emergency to address the health crisis. $1.2 million has been allocated in a response that includes mandatory fumigation of homes, a public education campaign in repelling mosquitoes and setting up tents for early detection and treatment” (18)
It is hoped that “Successful introduction of dengue immunization, alongside other prevention efforts, should help endemic countries to achieve the WHO objectives of reducing dengue morbidity by 25% and mortality by 50% by 2020.”(19)
Research:
- http://www.medicinenet.com/script/main/art.asp?articlekey=6564
- http://www.who.int/immunization/diseases/dengue/en/
- Http://www.passporthealthusa.com/destination-advice/peru/health-alerts/
- http://www.cdc.gov/dengue/epidemiology/index.html
- http://www.denguevirusnet.com/life-cycle-of-aedes-aegypti.html
- Http://www.sanofipasteur.com/en/articles/dengvaxia-world-s-first-dengue-vaccine-approved-in-mexico.aspx
- http://time.com/4296193/who-dengue-vaccine/
- http://www.hirunews.lk/131584/frances-dengue-vaccine-to-be-tested-for-suitability-in-sri-lanka
- http://www.sanofipasteur.us/about/vaccines
- http://www.channelnewsasia.com/news/asiapacific/historic-dengue-vaccine/2664344.html
- http://www.denguevaccines.org/vaccine-development
- http://www.star2.com/health/wellness/2016/04/24/is-the-world-finally-ready-to-fight-dengue/
- https://www.researchgate.net/profile/Simonetta_Viviani2/publication/230848750_Protective_efficacy_of_the_recombinant_live-attenuated_CYD_tetravalent_dengue_vaccine_in_Thai_schoolchildren_A_randomised_controlled_phase_2b_trial/links/02e7e515c1a310b42b000000.pdf
- http://www.sanofipasteur.com/en/articles/dengvaxia-world-s-first-dengue-vaccine-approved-in-mexico.aspx
- http://www.ncbi.nlm.nih.gov/pubmed/23773330
- http://www.sciencedirect.com/science/article/pii/S0264410X05009837
- http://news.berkeley.edu/2011/12/21/dengue/
- http://www.sciencedirect.com/science/article/pii/S1879625713000746
- http://perureports.com/2015/04/20/increase-in-dengue-cases-prompt-state-of-emergency-in-piura/
- http://www.scielosp.org/scielo.php?pid=S0042-96862005000400016&script=sci_arttext




